Most Popular Products
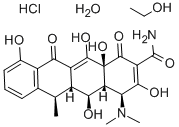
Fosaprepitant
Fosaprepitant Specification
- Shelf Life
- 2 years if stored properly
- Boiling point
- Not available (decomposes)
- Ph Level
- Not determined
- Poisonous
- No (when used as per pharmaceutical guidelines)
- Molecular Weight
- 1004.83 g/mol (dimeglumine salt)
- Molecular Formula
- C23H22F7N4O6PC2H6O5Mg
- Color
- White to off-white
- EINECS No
- none assigned
- Solubility
- Soluble in water
- Storage
- Store at 2-8C, protected from light
- Structural Formula
- Refer to product image or chemical database
- Loss on Drying
- 2.5%
- Particle Size
- Typically 90% < 10 microns
- HS Code
- 29339900
- Smell
- Odorless
- Heavy Metal (%)
- < 0.001%
- Melting Point
- 160-165C
- Medicine Name
- Fosaprepitant
- Chemical Name
- Fosaprepitant dimeglumine
- CAS No
- 172673-20-0
- Type
- Other
- Grade
- Other
- Usage
- Used as an antiemetic for prevention of chemotherapy-induced nausea and vomiting (CINV)
- Purity(%)
- >= 98%
- Appearance
- White to off-white powder
- Physical Form
- Solid
- Residue on Ignition
- 0.1%
- Regulatory Status
- USP/EP compliant batches available
- Packaging
- Double polyethylene bags, sealed in fiber drums
- Application
- Pharmaceutical intermediate, drug formulation
- Certificate of Analysis
- Provided with each batch
- Related Substances
- < 0.5%
- Endotoxin Level
- < 0.25 EU/mg
- Stability
- Stable under recommended storage conditions
- Identification Methods
- HPLC, NMR, IR
Fosaprepitant Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Supply Ability
- 2000 Kilograms Per Day
- Delivery Time
- 1 Days
About Fosaprepitant
Capitalise on the formidable efficacy of Fosaprepitant dimeglumine, a potent antiemetic designed for the prevention of chemotherapy-induced nausea and vomiting (CINV). Offered at an unbeatable price, this new pharmaceutical intermediate boasts an ineffable combination of >98% purity and exceptional stability under recommended storage conditions. Each batch is meticulously packaged in double polyethylene bags and fiber drums, accompanied by a Certificate of Analysis, ensuring regulatory compliance (USP/EP). Identified by advanced HPLC, NMR, and IR methods, Fosaprepitant features minimal related substances (<0.5%) and low endotoxin levels (<0.25 EU/mg), making it ideal for drug formulation and clinical applications.
Application Excellence of Fosaprepitant
Fosaprepitant is expertly formulated for use in pharmaceutical manufacturing and clinical drug development, targeting sites where high-grade antiemetic activity is required, particularly in oncology settings. Its features include stable chemical structure, rapid solubility in water, and fine powder consistency (90% < 10 microns). The products solid form and white to off-white appearance facilitate uniform blending and dissolution in drug formulations, supporting reproducible outcomes in machine-based applications for large-scale and custom pharmaceutical compounding.
Fosaprepitant Delivery, Payment, and Packaging Advantages
Experience swift rate-based delivery timelines with Fosaprepitant, tailored to meet urgent pharmaceutical needs. Packaging is secured via double polyethylene bags inside sealed fiber drums, safeguarding product integrity during freight and transit. Payment terms are flexible, accommodating secured transactions for distributors, exporters, and importers. Packaging details and freight costs are clearly communicated with each order, ensuring transparency and complete understanding of shipment conditions to guarantee the timely arrival of stable, high-quality batches.
Application Excellence of Fosaprepitant
Fosaprepitant is expertly formulated for use in pharmaceutical manufacturing and clinical drug development, targeting sites where high-grade antiemetic activity is required, particularly in oncology settings. Its features include stable chemical structure, rapid solubility in water, and fine powder consistency (90% < 10 microns). The products solid form and white to off-white appearance facilitate uniform blending and dissolution in drug formulations, supporting reproducible outcomes in machine-based applications for large-scale and custom pharmaceutical compounding.
Fosaprepitant Delivery, Payment, and Packaging Advantages
Experience swift rate-based delivery timelines with Fosaprepitant, tailored to meet urgent pharmaceutical needs. Packaging is secured via double polyethylene bags inside sealed fiber drums, safeguarding product integrity during freight and transit. Payment terms are flexible, accommodating secured transactions for distributors, exporters, and importers. Packaging details and freight costs are clearly communicated with each order, ensuring transparency and complete understanding of shipment conditions to guarantee the timely arrival of stable, high-quality batches.
FAQs of Fosaprepitant:
Q: How should Fosaprepitant be stored for optimal stability?
A: Fosaprepitant must be stored at temperatures between 2-8C, protected from light. This ensures the products stability throughout its 2-year shelf life and maintains its efficacy for pharmaceutical applications.Q: What identification methods are used to confirm Fosaprepitants authenticity?
A: Fosaprepitants authenticity is verified using advanced techniques such as HPLC, NMR, and IR, ensuring that each USP/EP-compliant batch meets stringent quality standards before distribution.Q: When will Fosaprepitant be delivered after placing an order?
A: Delivery times for Fosaprepitant are determined by the rate and transit options selected. Orders are dispatched quickly, with secure packaging and freight arrangements; exact timelines are outlined during the order process.Q: Where is Fosaprepitant commonly applied in pharmaceutical use?
A: Fosaprepitant is primarily used in hospital and clinical settings for drug formulation, specifically targeting chemotherapy-induced nausea and vomiting (CINV) as an effective antiemetic agent.Q: What is the process for receiving a Certificate of Analysis for Fosaprepitant?
A: A detailed Certificate of Analysis is provided with each batch of Fosaprepitant, outlining regulatory status, related substances, endotoxin level, purity, and other critical specifications as required for pharmaceutical documentation.Q: How does the packaging of Fosaprepitant ensure product integrity?
A: Fosaprepitant is packaged in double polyethylene bags, sealed inside fiber drums, which protect against moisture, contamination, and physical hazards during storage and transportation.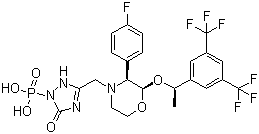
Price 100 INR/ Kilograms
- Minimum Order Quantity
- 1 Kilograms
- Supply Ability
- 2000 Kilograms Per Day
- Delivery Time
- 1 Days
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical API Category
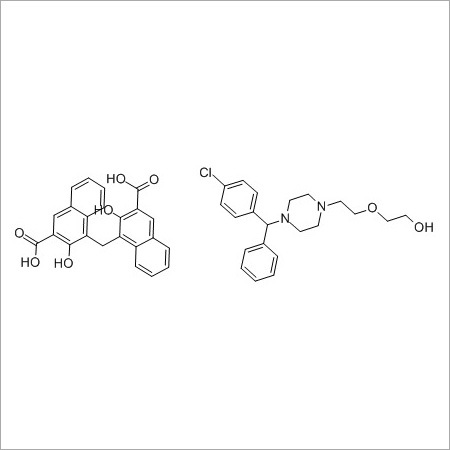
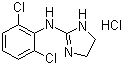
Clonidine hydrochloride
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Heavy Metal (%) : 0.001%
Physical Form : Solid
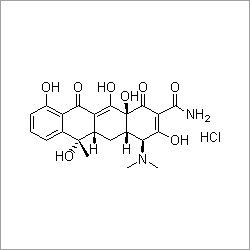
Doxycycline hydrochloride
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Heavy Metal (%) : 0.001%
Physical Form : Solid
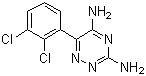
Lamotrigine
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Heavy Metal (%) : 0.001%
Physical Form : Solid
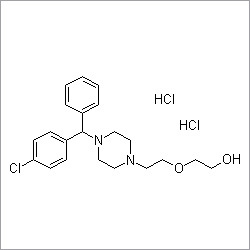
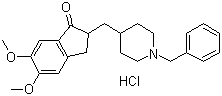
Donepezil hydrochloride
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Heavy Metal (%) : Not more than 0.001%
Physical Form : Powder
"We are into Supply of Pharmaceutical Raw Material / API". We only deal in Bulk Quantity. We do not deals in Pakistan and Bangladesh Countries.
 |
ANGLE BIO PHARMA
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |