Most Popular Products
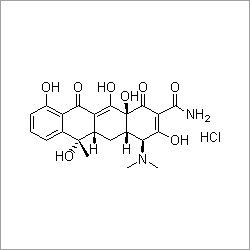
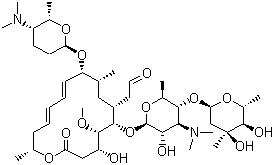
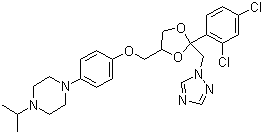
Everolimus
Everolimus Specification
- Poisonous
- Yes (handled as hazardous)
- Color
- White to off-white
- Heavy Metal (%)
- 0.001%
- Molecular Weight
- 958.2 g/mol
- Shelf Life
- 2 years when stored properly
- Boiling point
- Not available (decomposes before boiling)
- Solubility
- Practically insoluble in water; soluble in methanol, ethanol, and DMSO
- Structural Formula
- See image or C53H83NO14
- EINECS No
- None assigned
- Loss on Drying
- 0.5%
- Storage
- Store at 2-8C, dry and protected from light
- Smell
- Odorless
- Melting Point
- Approx. 184C
- HS Code
- 29349990
- Molecular Formula
- C53H83NO14
- Medicine Name
- Everolimus
- Chemical Name
- Everolimus
- CAS No
- 159351-69-6
- Type
- Other
- Grade
- Other
- Usage
- Used as an immunosuppressant and anticancer agent
- Purity(%)
- 98%
- Appearance
- White to off-white crystalline powder
- Physical Form
- Solid
- Assay Method
- HPLC
- Storage Conditions
- Keep container tightly closed in a cool, dry place
- Related Substances
- Total impurities: 1.0%
- Identification
- HPLC, IR, NMR, MS conform to standard
- Residual Solvents
- Complies with ICH guidelines
- Formulation
- Active pharmaceutical ingredient (API)
- Specific Optical Rotation
- +11 to +15 (c = 1, CHCl3)
Everolimus Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Supply Ability
- 2000 Kilograms Per Day
- Delivery Time
- 1 Days
About Everolimus
New Promotion: Grab Yours Everolimus, the noble and prime active pharmaceutical ingredient, stands out for its exquisite purity (98%), conforming to standard identification via HPLC, IR, NMR, and MS. Presented as a white to off-white crystalline powder, Everolimus satisfies stringent regulations for residual solvents and impurities (1.0%), making it a premier choice for immunosuppressant and anticancer applications. With specific optical rotation of +11 to +15 and a melting point near 184C, this odorless, hazardous solid is professionally supplied for medical use. Store tightly sealed in a cool, dry environment (2-8C) for optimal shelf life.
Area Of Application, Direction Of Use, Competitive Advantages
Everolimus is widely applied in transplant immunosuppression and cancer therapy due to its potent active pharmaceutical attributes. Administered under strict medical direction, Everolimus offers superior purity, minimal impurities, and excellent stability compared to alternatives. Its solubility in methanol, ethanol, and DMSO permits versatile formulation. The products compliance with ICH guidelines and prime storage conditions ensures reliable, consistent performance for healthcare professionals and patients.
Domestic Market, Handover, Certifications, FOB Port
Everolimus is supplied across major domestic markets with prompt handover and secure delivery arrangements from certified manufacturers, exporters, and distributors. The product meets regulatory standards, including GMP and ICH certifications, reflecting its noble quality. Delivery is handled from reliable FOB ports to ensure rapid fulfillment. Each supply batch undergoes thorough verification, enabling consistent and trustworthy product availability for national and international clients.
Area Of Application, Direction Of Use, Competitive Advantages
Everolimus is widely applied in transplant immunosuppression and cancer therapy due to its potent active pharmaceutical attributes. Administered under strict medical direction, Everolimus offers superior purity, minimal impurities, and excellent stability compared to alternatives. Its solubility in methanol, ethanol, and DMSO permits versatile formulation. The products compliance with ICH guidelines and prime storage conditions ensures reliable, consistent performance for healthcare professionals and patients.
Domestic Market, Handover, Certifications, FOB Port
Everolimus is supplied across major domestic markets with prompt handover and secure delivery arrangements from certified manufacturers, exporters, and distributors. The product meets regulatory standards, including GMP and ICH certifications, reflecting its noble quality. Delivery is handled from reliable FOB ports to ensure rapid fulfillment. Each supply batch undergoes thorough verification, enabling consistent and trustworthy product availability for national and international clients.
FAQs of Everolimus:
Q: How is Everolimus identified and tested for quality?
A: Everolimus is identified and tested using advanced methods such as HPLC, IR, NMR, and MS. The assay method is HPLC, confirming purity (98%) and ensuring all quality standards, including total impurities 1.0%, are met.Q: What is the primary usage of Everolimus?
A: Everolimus is primarily used as an immunosuppressant for transplant patients and as an anticancer agent, benefiting those requiring advanced therapeutics in specialized clinical settings.Q: When should Everolimus be stored for best shelf life?
A: Everolimus should be stored in a tightly closed container, in a dry place, protected from light, and at temperatures between 28C. These conditions guarantee a shelf life of up to two years.Q: Where are delivery and supply operations for Everolimus handled?
A: Delivery and supply of Everolimus are managed via certified distributors and suppliers, with handover typically arranged through reliable FOB ports, catering to both domestic and international markets.Q: What certifications does Everolimus comply with?
A: Everolimus complies with major international standards, including ICH guidelines for residual solvents and GMP certifications, ensuring product safety, efficacy, and regulatory compliance for medical applications.Q: How does the process of acquiring Everolimus work for buyers?
A: Buyers may contact authorized distributors, exporters, importers, manufacturers, or suppliers who then process the order, verify certifications, and arrange rapid delivery from designated FOB ports.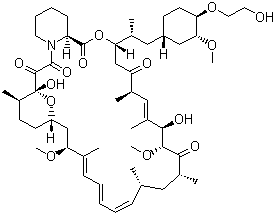
Price 100 INR/ Kilograms
- Minimum Order Quantity
- 1 Kilograms
- Supply Ability
- 2000 Kilograms Per Day
- Delivery Time
- 1 Days
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical API Category
Spiramycin
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Melting Point : 133135C (decomposes)
HS Code : 29419090
Diethylcarbamazine Citrate
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Melting Point : 208214C (decomposes)
HS Code : 29335990
Lovastatin
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Melting Point : 174C to 178C
HS Code : 29420090
Terconazole .
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Type : Other
Grade : Other
Melting Point : 128130C
HS Code : 29332990
"We are into Supply of Pharmaceutical Raw Material / API". We only deal in Bulk Quantity. We do not deals in Pakistan and Bangladesh Countries.
 |
ANGLE BIO PHARMA
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |